|
|
|
Atom is the smallest part of an element which can exist as a stable entity.
Molecule is a group of atoms, and this is the form that substances exist.
For examples, an element hydrogen dose not exist as "H" alone. It exist as "H2".
Water exist as "H2O" (two hydrogen atoms and one oxygen atom bonded together).
back to top
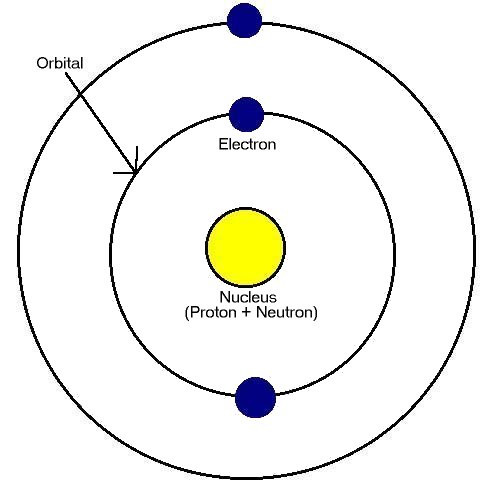
Structure of atoms
Three fundamental particles are electron, proton and neutron. Electrons surround the nucleus of the atom.
The nucleus, which contains protons and neutrons.
Their relative mass and charges are listed below
| Particle | Relative mass | Relative charge |
| electron | 0 | -1 |
| proton | 1 | +1 |
| neutron | 1 | 0 |
back to top
Ions
Ions are atom or group of atoms with an electric charge. They can be either positive or negative. Positive ions are called "Cation" and negative ions are called "Anion".
An easy way to remember these two ions
back to top
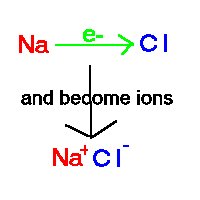
Ionic Bonding
is a complete transfer of electrons between atoms.
For Example Na + Cl ® Na+ Cl-
Covalent Bonding
is a force generated by sharing of electrons. Giant covalent bonding occurs in carbon compounds such as diamond and graphite. Generally, when covalent bonding is mentioned, it refers to simple covalent bonding.
Hydrogen Bonding and Vander Waals forces are intermolecular forces which occurs between molecules.
back to top
Orbitals.....shells and subshells
The term "orbital" is sometimes called "shell". Each shell can contain a certain number of electrons.
Each shell contains subshells. These subshells are designated by letter s, p, d and f. Each subshell has different energy, shape and orientation.
Therefore subshells contained in each shell will be
Now how do we know in what order the subshell are filled with electrons. The answer is, a small table show below can solve this problem.
So the order will be: 1s 2s 2p 3s 3p 4s
3d 4p (4s fills electron before 3d)
back to top
Amount of substances.....Avogadro's constant
Avogadro's constant (6.02x1024) is the number of molecules in 1 mole of a substance.
Now you will probably think "how about molecules like H2O and O2??"
Well the answer is easy. So for H2O = 1+1 (two hydrogens) +16 (one oxygen) = 18
Note: Relative atomic mass and relative molecular mass have NO UNITS!
Bondings
There are four types of bondings you have to remember
Metalic Bonding
occurs in metals (eg Mg, Cu). It can be described as "islands of cations in a sea of electrons".

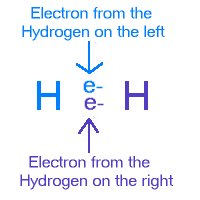
The small positive charge on the hydrogen atom is then attracted to a lone pair electron of an oxygen atom of another water molecule. This electrostatic attraction is the hydrogen bonding. Oxygen, Fluorine and Nitrogen always form hydrogen bond because of their high electronegativities.
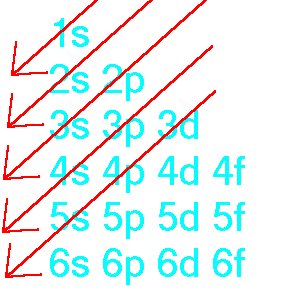 The red arrows show the order that electron is filled.
The red arrows show the order that electron is filled.
Now the question is how much is a mole??? This can be calculated from relative atomic mass shown in the periodic table.
For example, let's say you have 23g of Na (Sodium). If you look up the periodic table, it says 23 under Na, which means you have a mole of Na.
How about if you only have 12g of Na??? Well you can simply use the equation
 So the answer will be 12 / 23 = 0.523
So the answer will be 12 / 23 = 0.523
Therefore 12g of Na is 0.523 mole
Just substitute the "relative atomic mass" bit in the equation to "relative molecular mass".
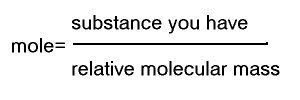
What is relative molecular mass then? It is simply the sum of relative atomic mass.
and for O2 = 16 +16 (two oxygens) = 32
| Contents & give your comment | Structure and Bonding | Inorganic Chemistry | Organic Chemistry | Physical Chemistry | Calculation | Techniques | Definitions | Periodic table | References |