|
|
|
back to top
CH2NH2 + H + ® CH2
NH3+
Oxidation: CH3CH2OH ® CH3CHO ® CH3COOH
(ethanol ® ethanal® ethanoic acid)
Reduction: CH3COOH ® CH3CH2OH
(ethanoic acid ® ethanol)
Acid + Alcohol ® Ester + water (This example is also known as esterification)
CH3CºN + H2O ® CH3CONH2 (ethanonitrile + water ® ethanoamide)
CH3CH2Br ® CH2=CH2 + HBr
CH3CH2OH ® CH2=CH2 + H2O
The reactions listed so far I believe are quite familiar for people who did their chemistry in junior high, high school or GCSE. Reactions after this point are the new bit for you (I hope).
Before we get into details of these reactions, first of all what are nucleophile, electrophile and free radicals??
OH-, and (:CºN (can be written as CN-) are examples of nucleophile with negative charge
CH3CH2(:NH2, H2O(water), NH3, and CH2=CH2 are examples of neutral nucleophile.
CH3+, H+, and NO2+ are examples of positive electrophile.
Br2 and HBr are examples of neutral electrophile.
When Cl2 is split up by UV, homolitic fission occurs and free radicals are formed. Cl-Cl® Cl* *Cl
CH3CH2Br + OH- ® CH3CH2OH + Br-
CH3CHO + HCN ® CH3CHCNOH
1)Making electrophile
2)The reaction
3)Leaving H+ react with AlCl4-
C2H4 +HBr ® CH3CH2Br
Overall reaction: CH4 + Cl2 ® CH3Cl + HCl
Initiation: Cl-Cl ® Cl* *Cl
Propagation:
CH4 + Cl* ® CH3 + HCl
Termination: (when all the free radicals are used up)
CH3* *Cl ® CH3Cl
Note: Cl* and Cl* can also form back to Cl-Cl but only happens on vessel wall.
Initiation
back to top
Geometrical isomers (cis and trans isomerism)
Geometrical isomers have the same structure and molecular formula but differ in the geometric arrangement.
Optical isomers
Optical isomers have the same molecular formula and structure but different arrangement in space, as the isomers are mirror image to each other and cannot be superimposed.
Types of reactions in organic chemistry
Acid donates H + and base accepts H +.
Reduction and Oxidation
Two molecules join to form a large molecule and a small molecule is evolved.
Breaking off a molecule by adding water.
Removal of a group from a molecule
Electrophilic substitution / addition
Free radical substitution / addition
Nucleophile can be negative or neutral
Electrophile can be positive or neutral
CH3Cl + AlCl3 ® CH3+
+ AlCl4-
AlCl4- + H+ ® AlCl3
+ HCl
It is divided into three stages: initiation, propagation, termination
CH3* + Cl2 ® CH3Cl + Cl*
CH3Cl + Cl* ® CH2Cl* + HCl
CH2Cl* + Cl2 ® CH2Cl2 + Cl*
CH3* *CH3 ® CH3-CH3
It is divided into three stages: initiation, propagation, termination
Propagation
Termination
Isomers
Isomers can be grouped as follow
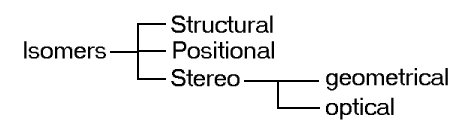
Note: Positional Isomer can also be grouped within structural isomer
Structural isomers
Structural isomers have the same molecular formulas but differ in structure.
For example: 2-methylbutane and 2,2-dimethylpropane. They are both C5H12 isomers.
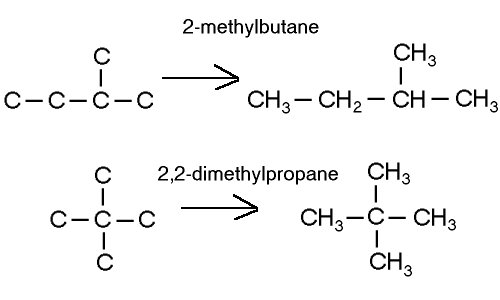
Positional isomers
Positional isomers have the same molecular formula and the same functional group but differ in the position of the functional group.
For example: propane-1-ol and propane-2-ol
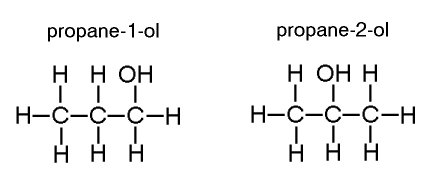
Stereo isomers
Stereo isomers have the same molecular formula and the same structure but have different arrangement in space.
Stereoisomers can be further subdivided into Geometrical and Optical.
For example: 1,2-dibromoethene
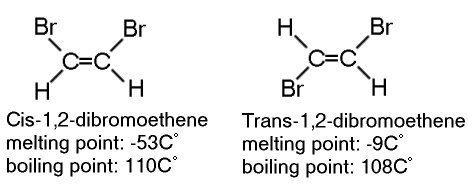
For example: butane-2-ol
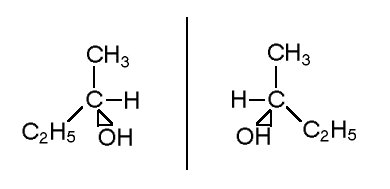
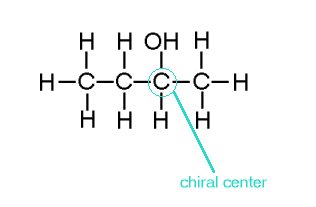
Optical isomers must at least have one chiral center. Chiral is a carbon with four atoms or groups attached to it which no matter how molecule is rotated, twisted or turned, it cannot superimpose to its mirror images.
Contents & give your comment
Structure and Bonding
Inorganic Chemistry
Organic Chemistry
Physical Chemistry
Calculation
Techniques
Definitions
Periodic table
References